EPR-72
Ib lub tshuab hluav taws xob yog ib yam ntawm cov hauv paus tseem ceeb uas muaj qhov hnyav thiab qhov tsis zoo. Nws tuaj yeem ua ob hom kev txav; ib qho yog txav mus los nyob ib puag ncig lub nucleus, thiab lwm qhov yog tig rau ntawm txoj kab hla dhau nws qhov chaw. Txij li kev txav ntawm cov hluav taws xob tsim lub sijhawm, cov dej ntws thiab lub sijhawm sib nqus tau tsim thaum lub zog txav. Hauv daim ntawv thov sib nqus sib nqus H, lub sijhawm sib nqus ntawm lub tshuab hluav taws xob ua zoo li tus pas nrig me me lossis rab koob. Txij li cov lej quantum tig ntawm lub tshuab hluav taws xob yog 1/2, lub tshuab hluav taws xob tsuas muaj ob txoj kev taw qhia nyob rau sab nraud sib nqus: ib qho yog sib npaug rau H, sib raug rau qib qis zog, lub zog yog -1/2gβH; ib qho yog antiparallel rau H, sib raug rau qib siab zog, lub zog yog +1/2gβH, thiab lub zog sib txawv ntawm ob qib yog gβH. Yog hais tias nyob rau hauv cov lus qhia ib puag ncig rau H, lub tshuab hluav taws xob sib nqus ntawm zaus v tau ntxiv kom tau raws li qhov xwm txheej ntawm hv = gβH, qib qis lub zog hluav taws xob nqus cov hluav taws xob nthwv dej hluav taws xob thiab dhia mus rau qib siab zog, uas yog hu ua hluav taws xob paramagnetic resonance .
Ub Cov khoom siv nrog cov tshuab hluav taws xob tsis sib xws (lossis ib qho hluav taws xob ib leeg) tshwm nyob rau hauv lub cev molecular orbital. Xws li cov dawb radicals (cov lwg me me muaj ib lub tshuab hluav taws xob ib leeg), dibasic thiab polybasic (cov lwg me me muaj ob lossis ntau dua ib lub tshuab hluav taws xob), triplet molecules (tseem muaj ob lub tshuab hluav taws xob nyob hauv lub cev molecular orbital, tab sis lawv nyob deb deb heev tsis ntev los no, muaj zog sib nqus sib cuam tshuam ntawm ib leeg, uas txawv ntawm ob lub hauv paus) thiab ntxiv rau.
Ub Cov xwm txheej nrog ib lub tshuab hluav taws xob tshwm sim hauv atomic orbitals, xws li alkali hlau atoms, hloov hlau ions (suav nrog pab pawg hlau, pab pawg palladium, thiab pab pawg platinum ions, uas tau tig rov qab ua tsis tau 3d, 4d, 5d lub plhaub), tsis tshua muaj lub ntiaj teb hlau ions (Nrog underfilled 4f plhaub) thiab ntxiv rau.
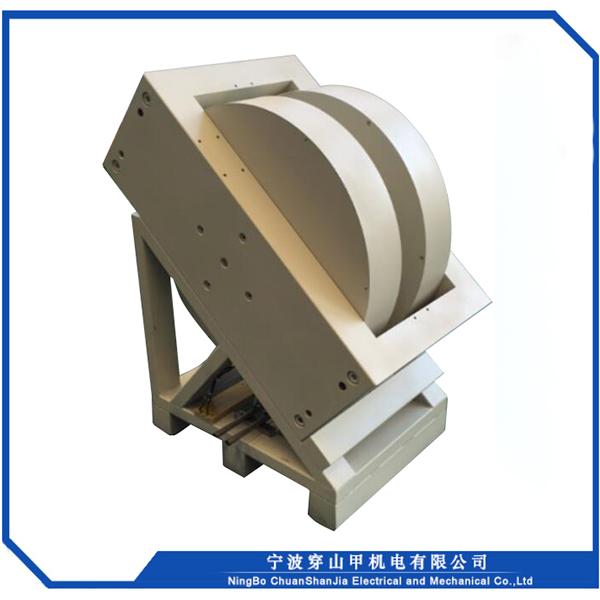
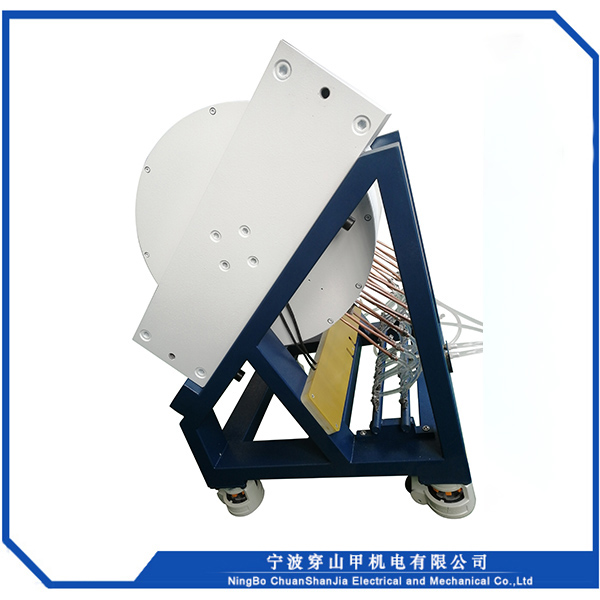
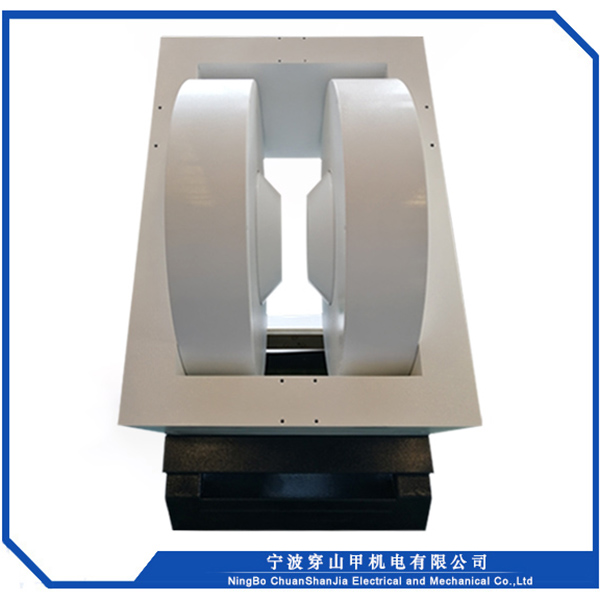
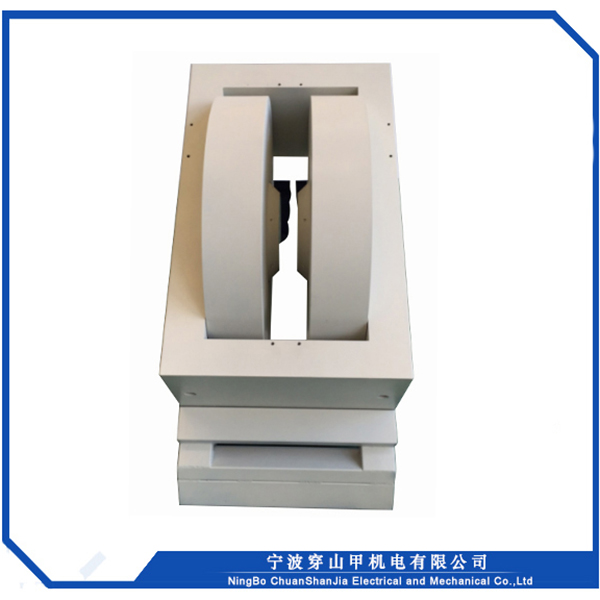
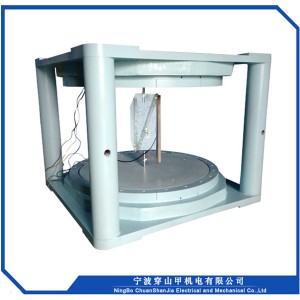
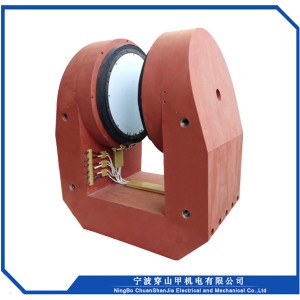
1, Sib nqus thaj tsam ntau: 0 ~ 18000 Gauss kho tsis tu ncua
2, Lub taub hau sib nrug : 72mm
3, Txoj kev txias : dej txias
4, Qhov hnyav tag nrho : <2000kg
Tau tuaj yeem ua raws li tus neeg yuav tsum tau ua